Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Are DHEAS Levels at Initiation of Ovarian Stimulation Different Between Pregnant and Non-pregnant after In-Vitro-Fertilization? Α Systematic Review and Meta-analysis
*Corresponding author:Viktoria Christoforaki MD MSc, Clinical Fellow in Reproductive Medicine, Unit for Human Reproduction, 1st Department of Obstetrics and Gynaecology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Received:December 15, 2022; Published:January 09, 2023
DOI: 10.34297/AJBSR.2023.17.002398
Abstract
The aim of this study was to evaluate whether dehydroepiandrosterone sulphate (DHEAS) levels at initiation of ovarian stimulation are different between pregnant and non-pregnant women after IVF. A systematic review/meta-analysis showed that no significant difference in DHEAS levels at initiation of ovarian stimulation was observed in women without polycystic ovarian syndrome (PCOS) who achieved or not clinical pregnancy [standardised mean difference (SMD): -0.15, 95%CI: -0.47 to +0.16, five studies, 2448 patients]. Similarly, no significant difference in DHEAS levels at initiation of ovarian stimulation was observed in women in the general population (SMD: +0.37, 95%CI: -0.25 to +0.99, single study, 53 patients). In expected poor responders, significantly higher DHEAS levels at initiation of ovarian stimulation were observed in patients who achieved clinical pregnancy compared with those who did not (SMD: +0.87, 95%CI: +0.32 to +1.41, single study, 129 patients). Similar DHEAS levels at initiation of ovarian stimulation are present between patients who achieve or not clinical pregnancy in the general population as well as in women without PCOS. However, there is weak evidence to suggest that higher DHEAS levels at initiation of ovarian stimulation are present in poor responders who achieve clinical pregnancy compared with those who did not.
Keywords: Androgen, Pregnancy, In-vitro fertilization, IVF, Assisted reproductive technology
Abbreviations:DHEAS: Dehydroepiandrosterone Sulphate; PCOS: Polycystic Ovarian Syndrome; SMD: Standardised Mean Difference
Introduction
Androgens are involved in endometrial physiology since their receptors are present in all endometrial cell types [1]. Effective decidualization, a decisive step towards normal uncomplicated pregnancy, is controlled by a proper androgenic environment. This is guided by endogenous signalling that up- or down-regulate androgen receptors (AR) [2]. Androgens are abundant in the granulosa cells of healthy antral and pre-antral follicles. Furthermore, human granulosa cells carry AR and possess sulphatise activity that allows them to use dehydroepiandrosterone sulphate (DHEAS) for testosterone, androstenedione, and oestrogen production [3].
In the primate ovary, androgens, especially DHEAS and testosterone act as critical regulators of follicular development by augmenting the expression of insulin growth factor 1 (IGF1) and enhance follicular recruitment by increasing the expression of follicle stimulating hormone (FSH) receptors on the granulosa cells [4-6]. DHEAS and its inactive form DHEA are produced mostly by the adrenal glands and are the most abundant steroids in the plasma, providing large amounts of a substrate which is converted to other androgens and oestrogens [3,7,8]. The advantage of DHEAS over other androgens is that DHEAS is produced by DHEA only in target tissues that provide the necessary enzymatic environment, thus eliminating potential side effects from androgen and oestrogen action systematically [8].
Several studies have examined the association between endogenous DHEAS levels and achievement of pregnancy after in-vitro fertilization (IVF), producing conflicting, however, results [9-12]. The aim of this systematic review and meta-analysis was to answer the following research question: are DHEAS levels at initiation of ovarian stimulation different between pregnant and non-pregnant women after IVF?
Materials and Methods
Identification Of Literature
A computerized literature search in MEDLINE, CENTRAL and randomized controlled trials (RCTs) registries covering the period until March 2022 was performed independently by two reviewers (V.E.C and E.M.K), aiming to identify all available studies evaluating the research question of interest: Are DHEAS levels at initiation of ovarian stimulation different between pregnant and non-pregnant women after IVF? For this purpose, several keywords were used, shown in Supplementary Table 1.

Table S1: Search strategy used for the identification of eligible studies (these terms were search as “free text terms”).
Additionally, the citation lists of all relevant publications and review articles were hand-searched. No language limitations were applied. Institutional Review Board was not obtained as previously published data were used. This study followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) guidelines [13] (PROSPERO registration number: CRD42022315804) (Supplementary Table 2), (Tables 1-4), (Figures 1-3).
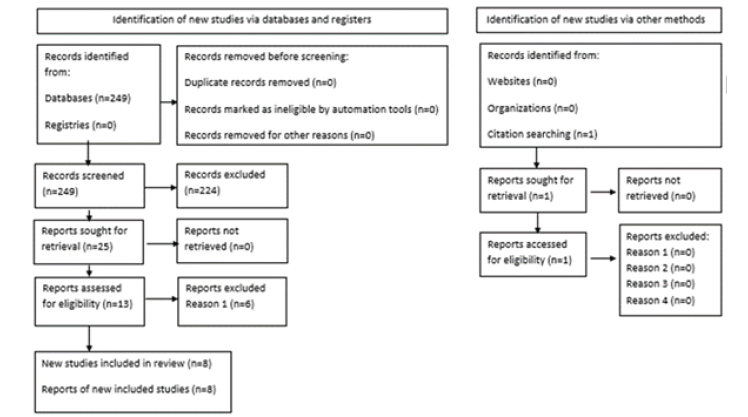
Figure 1: PRISMA flow diagram detailing selection of studies for inclusion in the systematic review/meta-analysis.
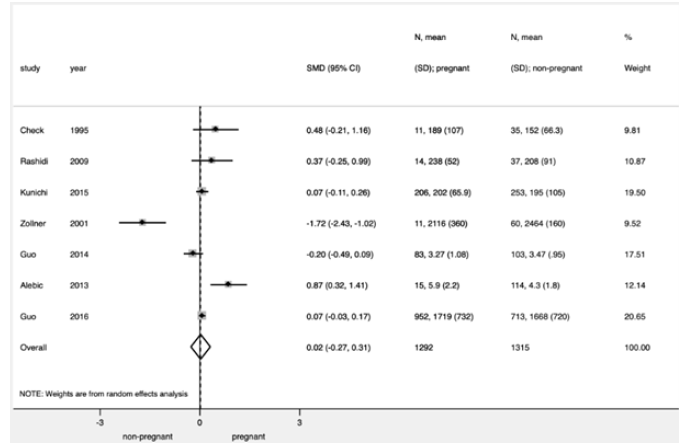
Figure 2:Difference in DHEAS levels at initiation of ovarian stimulation in patients who achieved clinical pregnancy after IVF/ICSI compared with those who did not.
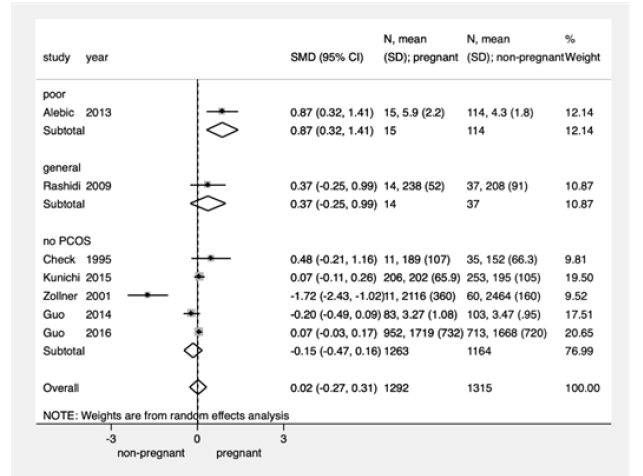
Figure 3:Difference in DHEAS levels at initiation of ovarian stimulation in patients who achieved clinical pregnancy after IVF/ICSI compared with those who did not, performed by subgroup analysis by patient population.
Table S2: From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71.
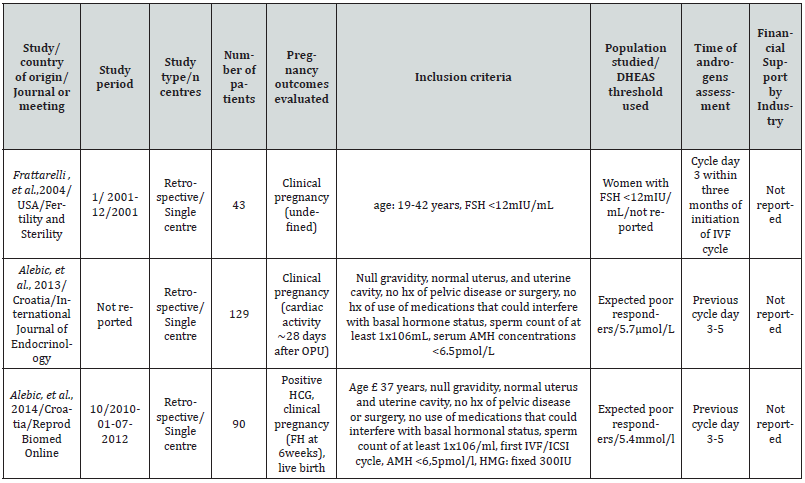
Table 1:Characteristics of eligible studies evaluating the probability of pregnancy after IVF/ICSI in patients with high or low DHEAS levels at initiation of stimulation (A).
Note*: IVF: In-vitro fertilization, ICSI: intra-cytoplasmic sperm injection, SEI: successful embryo implantation, FEI: failed embryo implantation, OPU: oocyte pick-up, Hx: history, FH: fetal heart, PCOS: polycystic cystic ovarian syndrome, HCG: human chorionic gonadotropin, DHEAS: dehydroepiandrosterone sulphate.
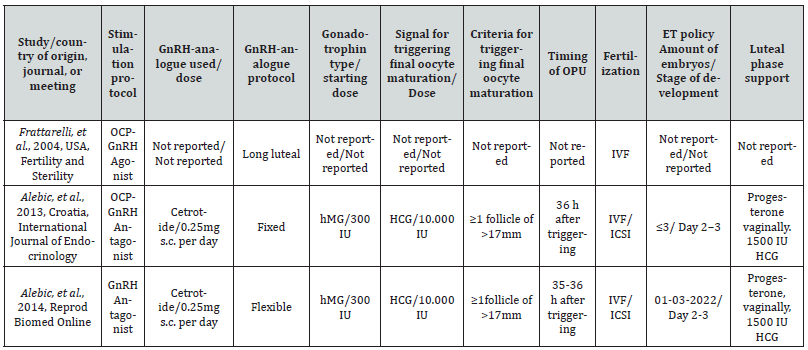
Table 2:Characteristics of eligible studies evaluating the probability of pregnancy after IVF/ICSI in patients with high or low DHEAS levels at initiation of stimulation (B).
Note*: ET: embryo transfer, OCP: oral contraceptives, OPU: oocyte pick-up, GnRH: Gonadotrophin releasing hormone, hMG: human chorionic gonadotrophin, rFSH: recombinant Follicle stimulating Hormone (FSH), IVF: in-vitro fertilization, ICSI: intracytoplasmic sperm injection SC: subcutaneous; IM: intramuscular
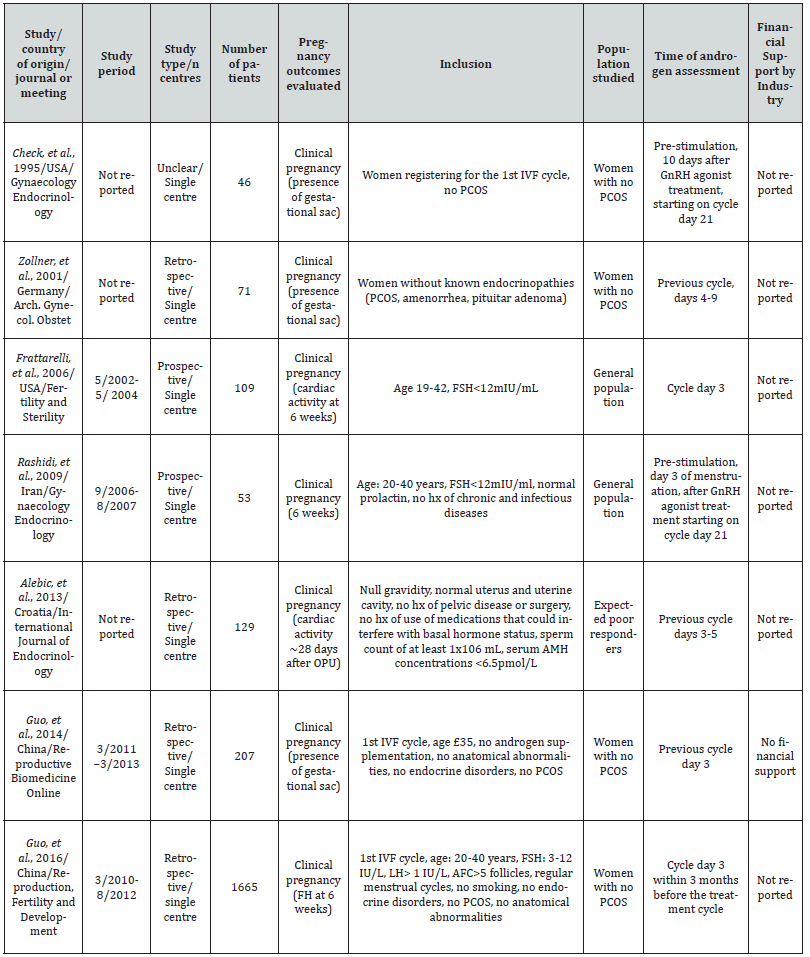
Table 3:Characteristics of eligible studies evaluating the difference in DHEAS levels at initiation of ovarian stimulation in patients who achieved pregnancy or not after IVF/ICSI (A).
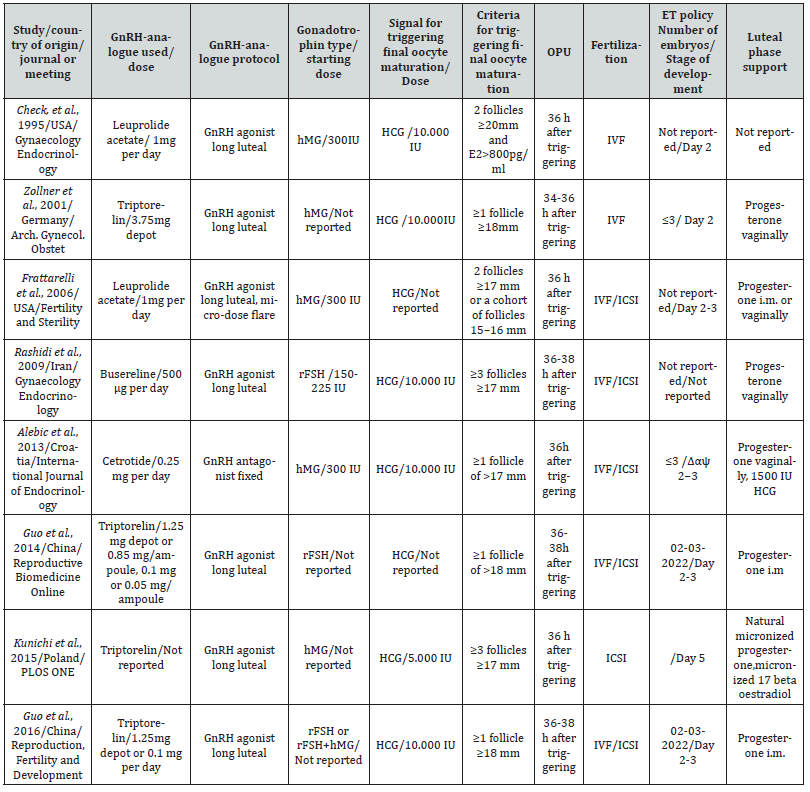
Table 4:Characteristics of eligible studies evaluating the difference in DHEAS levels at initiation of ovarian stimulation in patients who achieved pregnancy or not after IVF/ICSI (B).
Selection Of Studies
Criteria for inclusion/exclusion of studies were established prior to literature search. Eligible studies for inclusion in the current systematic review and meta-analysis were prospective or retrospective studies that had assessed serum DHEAS levels at initiation of ovarian stimulation in women subjected to IVF by either conventional insemination or intracytoplasmic sperm injection (ICSI). Ovarian stimulation for multi-follicular development should have been performed using gonadotrophin releasing hormone (GnRH) analogues and gonadotrophins. Selection of studies was performed independently by two of the reviewers (V.E.C and E.M.K) and any disagreement was resolved by discussion..
Data Extraction
Data extraction was performed independently by two of the authors (V.E.C and E.M.K). The following data were extracted from each of the eligible studies: demographic (year of publication, country, study period, number of patients included), methodological (type of study), procedural [whether financial support was declared, type of GnRH analogue and protocol used for luteinizing hormone (LH) surge inhibition, type and starting dose of gonadotrophin administered for ovarian stimulation, type and dose of medication used for triggering final oocyte maturation, criteria used for triggering final oocyte maturation, type of fertilization, day of embryo transfer, type of luteal support, endometrial preparation]. Any disagreement between the two authors responsible for data extraction was resolved by discussion. In case of missing data or ambiguities in study design or trial conduction, the study authors were contacted by e-mail to request additional information. Primary outcome was the difference in DHEAS levels at initiation of ovarian stimulation between pregnant and non-pregnant women after IVF.
Risk Of Bias and Study Quality Assessment/
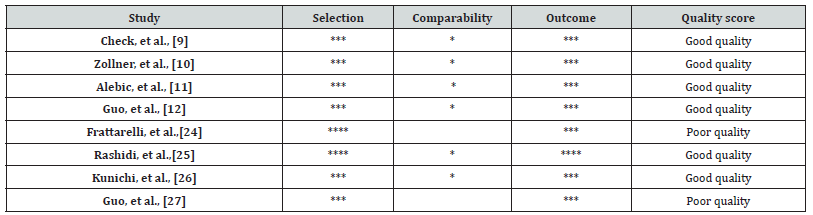
The quality of the eligible studies was assessed with the use of the Newcastle-Ottawa quality assessment scale (NOS) [14] adapted for the specific research questions allowing up to 9 stars (Supplementary Table 3). Two of the authors (V.E.C and E.M.K) independently evaluated the overall quality of the evidence. Any disagreement was resolved by discussion.
Subgroup Analysis
The influence of population type (general population, expected poor responders, women without PCOS) was explored by performing subgroup analysis.
Statistical Analysis
Pooling of data was performed using the inverse variance method Hedges, et al., in the case of fixed effects model, and the DerSimonian and Laird method [15], in the case of the random effects model. When the outcome of interest was of a continuous nature, the differences were pooled across the studies which provided information on this outcome, resulting in a standardized mean difference (SMD) with 95% confidence interval (CI). Study-to-study variation was assessed by using the Chi2 statistic (the hypothesis tested was that the studies are all drawn from the same population, i.e., from a population with the same effect size). In addition, the use of the I2 statistic was employed to indicate heterogeneity between studies that could not be attributed to chance, with I2 ≥40% indicating significant heterogeneity [16]. A fixed effects model was used, where no heterogeneity was present, while in the presence of significant heterogeneity, a random effects model was applied. The presence of publication bias was tested by using the Harbord- Egger’s test [17]. Statistical significance was set at a p-level of 0.05. Meta-analysis of weighted average effect sizes was performed using STATA v14.0 (Stata Corp. 2015. Stata Statistical Software: Release 14. College Station, TX: Stata Corp LP).
Results
Identification of Literature
Literature search yielded 249 studies, the screening of which by study title resulted in 25 potentially eligible publications. Twelve out of these 25 studies were excluded after reading their abstracts, while the remaining 13 studies were further evaluated by retrieving their full text. Six out of these 13 studies were excluded since they did not provide data to answer the research question [18-23]. After hand search of the citation lists of all relevant publications and review articles, one additional study was found to provide data that answered the research question [24]. Eight studies were eligible for the systematic review (2747 patients) [9-12,24-27], assessing the difference in DHEAS levels between pregnant and non-pregnant women after IVF. A PRISMA flow diagram detailing selection of studies for inclusion is presented in Figure 1.
Systematic Review
Eight studies (two prospective, five retrospective and one of unclear design) published between 1995 and 2016 were eligible for the systematic review, including a total of 2747 patients. Characteristics of the studies included in the systematic review are presented in Tables 1-2. Most of the studies were of “good quality” as assessed by NOS Supplementary Table 2. Number of included patients ranged from 46 to 1665 (median=120). Three studies were conducted in Asia (n=1925), three studies in Europe (n=659) and two studies in the USA (n=163). Five studies provided data regarding the research question in women without PCOS (n=2448), two studies in the general population [where normal, high (women with PCOS) and poor responders were included] (n=170) and one study in expected poor responders (n=129).
As shown in Table 1, all studies provided data for clinical pregnancy, defined as the presence of intrauterine sac at 6-8 weeks gestation. Power analysis was performed in one of the two prospective studies.
As shown in Table 2, to inhibit premature LH surge, GnRH agonists were used in seven out of the eight studies, and GnRH antagonists were used in the remaining study. Ovarian stimulation was performed with human menopausal gonadotrophins (hMG) in five studies, with the use of recombinant FSH (rFSH) in two studies and with either rFSH or rFSH and hMG in a single study. Human chorionic gonadotrophin (hCG) was used to trigger final oocyte maturation in all studies Table 2. Oocyte retrieval was performed 34-38h after hCG administration in all studies.
Luteal phase was supported with vaginal progesterone in four studies, with intramuscular progesterone in two studies and either with intramuscular or vaginal progesterone in the remaining study. In addition to progesterone, estrogens or hCG were added in a single study. No information was present regarding luteal phase supplementation in a single study Table 2.
Meta-Analysis
Seven out of the eight studies included in the systematic review, assessing the difference in DHEAS levels at initiation of ovarian stimulation between pregnant and non-pregnant women after IVF provided data to answer the research question and were included in the meta-analysis. Harbord-Egger’s test was not performed due to the low number of studies in the groups evaluated [16].
Main Analysis
No significant difference in DHEAS levels at initiation of ovarian stimulation was observed between pregnant and non-pregnant women after IVF (SMD: +0.02, 95% CI: -0.27 to +0.31, p=0.88, seven studies, 2630 patients) (Figure 2) [9-11,25-27].
Subgroup Analysis According to The Type of Population
Expected Poor Responders
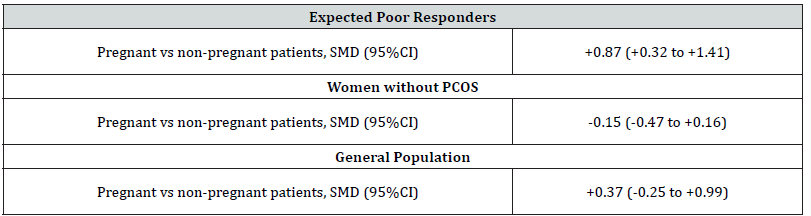
Table S4:Association between dehydroepiandrosterone sulphate (DHEAS) levels at initiation of ovarian stimulation and the achievement of pregnancy (clinical pregnancy) in patients undergoing IVF/ICSI stratified by type of population.
Note*: Results are presented as SMD (95% confidence interval) for the eligible studies assessing the difference in DHEAS levels at initiation of ovarian stimulation between patients who achieved or not pregnancy (clinical pregnancy) after IVF. Cells in bold indicate statistical significance while cells are left blank where no relevant information s present.
Significantly higher DHEAS levels at initiation of ovarian stimulation were observed in women who achieved clinical pregnancy compared with those who did not after IVF (SMD: +0.87, 95% CI: +0.32 to +1.41, p=0.002, single study, 129 patients) [11] (Supplementary Table 4).
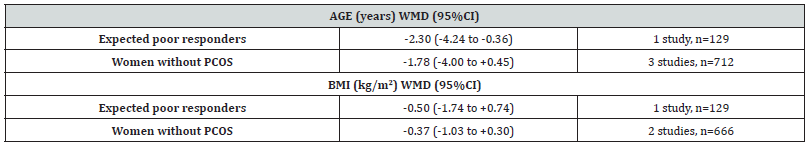
Although no significant difference was observed in body mass index (BMI) in women who achieved clinical pregnancy compared with those who did not, this was not the case regarding age which showed that women who achieved clinical pregnancy after IVF/ ICSI were significantly younger compared with those who did not (Supplementary Table 5) [11].
Note*: Results are presented as WHD (95% confidence interval). Cells in bold indicate statistical significance while cells are left blank where no relevant information is present
Women Without PCOS
No significant difference in DHEAS levels at initiation of ovarian stimulation was observed between pregnant and non-pregnant women after IVF (SMD: -0.15, 95% CI: -0.47 to +0.16, p=0.35, five studies, 2448 patients) Supplementary Table 4 & Figure 3 [9,10,12,26,27]. In these studies, no significant difference was observed in age or BMI between women between pregnant and non-pregnant women after IVF Supplementary Table 4.
General Population
No significant difference in DHEAS levels at initiation of ovarian stimulation was observed between pregnant and non-pregnant women after IVF (SMD: +0.37, 95% CI: -0.25 to +0.99, p=0.25, single study, 53 patients) Supplementary Table 4 & Figure 3 [28].
Discussion
This systematic review and meta-analysis, including eight eligible studies and 2747 women, showed that similar DHEAS levels are present at initiation of ovarian stimulation between women who achieve or not clinical pregnancy after IVF. This is true both for women without PCOS as well as for women in the general population. However, there is weak evidence to suggest that higher DHEAS levels at initiation of ovarian stimulation are present in poor responders who achieve clinical pregnancy compared with those who did not. The underlying mechanism regarding the increased DHEAS levels at initiation of ovarian stimulation observed in expected poor responders who achieved clinical pregnancy compared to those who did not remains unclear. It should be, however, taken into consideration that women who achieved clinical pregnancy were significantly younger compared with those who did not. It is well known that DHEAS levels decrease profoundly with age [29,30], which is negatively associated with the achievement of pregnancy [31,32]. Thus, it could be argued that the positive association between DHEAS levels at initiation of ovarian stimulation and the achievement of pregnancy might be explained by female age, although this requires further investigation.
It has been shown that DHEAS levels play an important role in folliculogenesis and follicular development, by stimulating the initiation of primordial follicles and enhancing the development of pre-antral and early antral follicles [33]. Furthermore, in studies performed in rats with diminished ovarian reserve, levels of atresia were reduced after DHEAS treatment [34]. In addition, a positive correlation between DHEAS levels in the follicular fluid and clinical pregnancy as well as live birth rates has been reported in non- PCOS women [35]. On the other hand, DHEAS supplementation prior to IVF in poor responders has been associated with improved aneuploidy rates [36], whereas no significant difference was observed in the number of oocytes retrieved, clinical pregnancy and live birth rates compared to no treatment [37,38]. Even though most of the studies included in the current systematic review and meta-analysis where of good quality, as assessed by the relevant quality tools, most of these studies were of retrospective nature with considerable heterogeneity regarding patient population and type of ovarian stimulation protocol used. Furthermore, although a lack of difference in the comparisons performed may represent a true lack of difference, it may also reflect a type b error due to the insufficient number of patients included in the eligible studies.
Conclusion
In conclusion, the present systematic review and meta-analysis showed similar DHEAS levels at initiation of ovarian stimulation between women who achieved pregnancy after IVF/ICSI compared with those who did not. This is true both for women without PCOS as well as for women in the general population. However, there is weak evidence to suggest that higher DHEAS levels at initiation of ovarian stimulation are present in poor responders who achieve clinical pregnancy compared with those who did not. If this finding is confirmed in future studies, it highlights the importance of taking into consideration DHEAS levels at initiation of ovarian stimulation in expected poor responders in case DHEA administration is proposed, since a differential effect of DHEA, depending on DHEAS levels might be present.
Declaration of Interest
None.
References
- Mertens HJ, MJ Heineman, J Koudstaal, P Theunissen, JL Evers, et al. (1996) Androgen receptor content in human endometrium. Eur J Obs Gyn Rep Biol 70(1): 11-13.
- Simitsidellis I, PTK Saunders, DA Gibson (2018) Androgens and endometrium: new insights and new targets. Mol Cell Endocrinol 465: 48-60.
- Bonser J, J Walker, A Purohit, MJ Reed, BV Potter, et al. (2000) Human granulosa cells are a site of sulphatase activity and are able to utilize dehydroepiandrosterone sulphate as a precursor for oestradiol production. J Endocrinol 167(3): 465-471.
- Vendola K, J Zhou, J Wang, OA Famuyiwa, M Bievre et al. (1999) Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 61(2): 353-357.
- Weil S, K Vendola, J Zhou, CA Bondy (1999) Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84(8): 2951-2956.
- Astapova O, BMN Minor, SR Hammes (2019) Physiological and Pathological Androgen Actions in the Ovary. Endocrinology 160(5): 1166-1174.
- Haning RV, RJ Hackett, RI Boothroid, JA Canick (1990) Steroid sulphatase activity in the human ovarian corpus luteum, stroma, and follicle: comparison to activity in other tissues and the placenta. J Steroid Bio 36(1-2): 175-179.
- Labrie F, V Luu The, C Labrie, A Belanger, J Simard, et al. (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24(2): 152-182.
- Check JH, A Nazari, C Dietterich (1995) Comparison of androgen levels in conception vs. non-conception cycles following controlled ovarian stimulation using the luteal phase gonadotropin-releasing hormone agonist protocol. Gynecol Endocrinol 9(3): 209-214.
- Zollner U, K Lanig, T Steck, J Dietl (2001) Assessment of endocrine status in patients undergoing in-vitro fertilization treatment. Is it necessary? Arch Gynecol Obstet 265(1): 16-20.
- Alebić M, N Stojanović, M Zuvić Butorac (2013) The IVF Outcome Counseling Based on the Model Combining DHEAS and Age in Patients with Low AMH Prior to the First Cycle of GnRH Antagonist Protocol of Ovarian Stimulation. Int J Endocrinol 2013: 637919.
- Guo J, Q Zhang, Y Li, J Huang, W Wang, et al. (2014) Predictive value of androgens and multivariate model for poor ovarian response. Rep Biomed Onl 28(6): 723-732.
- Page MJ, JE McKenzie, PM Bossuyt, I Boutron, TC Hoffmann, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71.
- Wells G, B Shea, DO Connell, J Peterson, V Welch, et al. (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute.
- DerSimonian R, N Laird (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3): 177-188.
- Higgins J, J Thomas, J Chandler, M Cumpston, T Li, et al. (2020) Cochrane Handbook for Systematic Reviews of Interventions. Recommendations on testing for funnel plot asymmetry.
- Harbord RM, M Egger, JA Sterne (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25(20): 3443-3457.
- Frattarelli JL, EH Peterson (2004) Effect of androgen levels on in vitro fertilization cycles. Fertil Steril 81(6): 1713-1714.
- Orvieto R, V Yulzari Roll, A La Marca, J Ashkenazi, B Fisch, et al. (2005) Serum androgen levels in patients undergoing controlled ovarian hyperstimulation for in vitro fertilization cycles. Gynecol Endocrinol 21(4): 218-222.
- Alebić M, N Stojanović (2014) Dehydroepiandrostendione sulphate and prediction of live birth after IVF in young women with low anti-Müllerian hormone concentration. Reprod Bio Onl 28(2): 191-197.
- Abide Yayla C, E Ozkaya, S Kayatas Eser, I Sanverdi, B Devranoglu, et al. (2018) Association of basal serum androgen levels with ovarian response and ICSI cycle outcome. Ir J Med Sci 187(2): 409-415.
- Findeklee S, P Sklavounos, L Stotz, RM Sima, I Iordache et al. (2019) Influence of androgen levels on conception probability in patients undergoing fertility treatment: a retrospective cohort study. Arch Gyn Obs 299(5): 1481-1485.
- Chimote BN, NM Chimote (2021) Dehydroepiandrosterone sulphate (DHEAS) concentrations stringently regulate fertilisation, embryo development and IVF outcomes: are we looking at a potentially compelling 'oocyte-related factor' in oocyte activation? J Assist Rep Genet 38(1): 193-202.
- Frattarelli JL, MD Gerber (2006) Basal and cycle androgen levels correlate with in vitro fertilization stimulation parameters but do not predict pregnancy outcome. Fertil Steril 86(1): 51-57.
- Hossein Rashidi B, Hormoz B, E Shahrokh Tehraninejad, Shariat M, Mahdavi A, et al. (2009) Testosterone and dehydroepiandrosterone sulphate levels and IVF/ICSI results. Gynecol Endocrinol 25(3): 194-198.
- Kunicki M, K Lukaszuk, G Jakiel, J Liss (2015) Serum dehydroepiandrosterone sulphate concentration is not a predictive factor in IVF outcomes before the first cycle of GnRH agonist administration in women with normal ovarian reserve. PLoS One 10(3): e0118570.
- Guo J, Q Zhang, Y Li, W Wang, D Yang, et al. (2016) Low level of basal testosterone: a significant risk factor for poor oocyte yield after ovulation induction. Reprod Fertil Dev 28(3): 286-292.
- Rashidi BH, B Hormoz, E Shahrokh Tehraninejad, M Shariat, A Mahdavi, et al. (2009) Testosterone and dehydroepiandrosterone sulphate levels and IVF/ICSI results. Gynecol Endocrinol 25(3): 194-198.
- De Pergola G, VA Giagulli, G Garruti, MR Cospite, F Giorgino, et al. (1991) Low dehydroepiandrosterone circulating levels in premenopausal obese women with very high body mass index. Metabolism 40(2): 187-190.
- Sulcova J, M Hill, R Hampl, L Starka (1997) Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol 154(1): 57-62.
- George K, MS Kamath (2010) Fertility and age. J Hum Reprod Sci 3(3): 121-123.
- Provost MP, KS Acharya, CR Acharya, JS Yeh, RG Steward, et al. (2016) Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril 105(3): 663-669.
- Narkwichean A, K Jayaprakasan, WE Maalouf, JH Hernandez Medrano, et al. (2014) Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod 29(1): 146-154.
- Hassa H, Y Aydin, O Ozatik, K Erol, Y Ozatik, et al. (2015) Effects of dehydroepiandrosterone (DHEA) on follicular dynamics in a diminished ovarian reserve in vivo model. Syst Biol Rep Med 61(3): 117-121.
- Chimote NM, NM Nath, NN Chimote, BN Chimote (2015) Follicular fluid dehydroepiandrosterone sulfate is a credible marker of oocyte maturity and pregnancy outcome in conventional in vitro fertilization cycles. J Hum Reprod Sci 8(4): 209-213.
- Gleicher N, DH Barad (2011) Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Rep Biol Endocrinol 9: 67.
- Neves AR, P Montoya Botero, NP Polyzos (2022) Androgens and diminished ovarian reserve: the long road from basic science to clinical implementation. A comprehensive and systematic review with meta-analysis. Am J Obs Gyn 227(3): 401-413 e418.
- Wang Z, A Yang, H Bao, A Wang, X Deng, et al. (2022) Effect of dehydroepiandrosterone administration before in vitro fertilization on the live birth rate in poor ovarian responders according to the Bologna criteria: A randomised controlled trial. BJOG 129(7): 1030-1038.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.